About Kedrion Biopharma
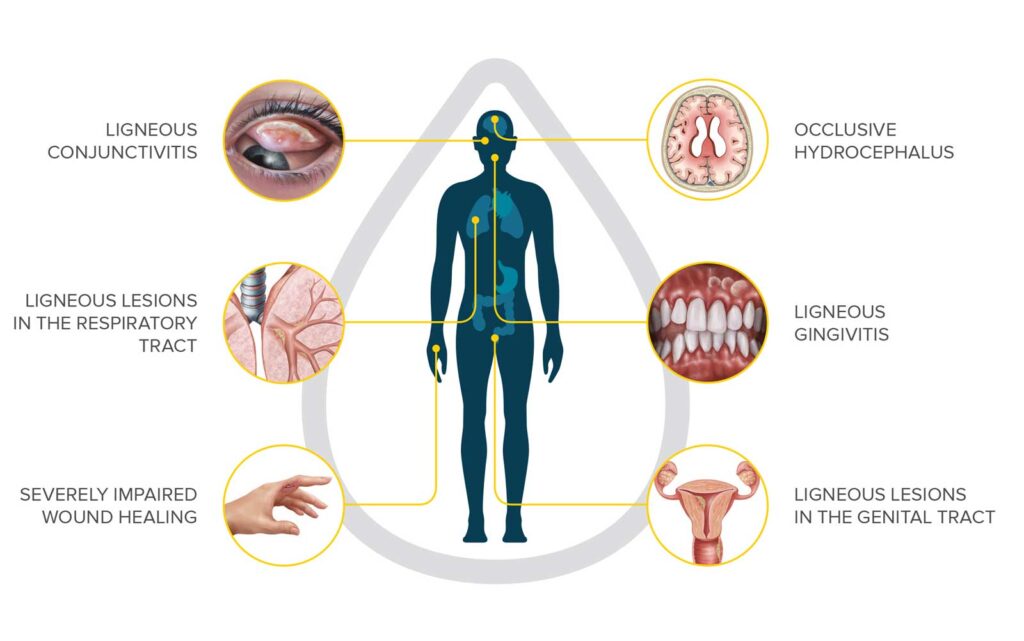
Kedrion, and its United States subsidiary Kedrion Biopharma, is an international company based in Italy with a long history of supporting patients with blood disorders, with a focus on rare and debilitating disorders. Kedrion has long been involved with the Plasminogen Deficiency community through the development of their Plasminogen Ophthalmic Solution, an eye drop medication to treat ligneous conjunctivitis, the most common symptom of plasminogen deficiency. This product is not yet approved by the FDA or commercially available, though a study evaluating its use is ongoing. In June of 2021, Kedrion purchased the license to Ryplazim, a plasma-derived human plasminogen concentrate indicated for the treatment of patients with plasminogen deficiency type 1 (hypoplasminogenemia), also known as C-PLGD.
“The most important mission at Kedrion Biopharma is to improve the lives of people with rare and serious diseases,” said Kedrion Biopharma Chief Executive Officer, Val Romberg. “As the newest addition to our growing portfolio of products, Ryplazim is an excellent example of that dedication. An ultra-orphan therapy, Ryplazim meets an urgent unmet medical need for people who face plasminogen deficiency type 1, a potentially devastating, but treatable, medical condition. We are pleased and gratified to be in a position now to help these patients.”
The Board of the Plasminogen Deficiency Foundation has had the opportunity to work with the leadership team at Kedrion to provide the patient perspective on plasminogen deficiency, and the multitude of ways that patients need support in living with this disorder. Below are questions and answers about the launch of Ryplazim, summarized from our conversations with the leadership team at Kedrion. We look forward to continuing this relationship in support of PLGD patients everywhere.
What is Ryplazim?
Ryplazim (pronounced RIP-la-zeem) is a new treatment for patients with plasminogen deficiency type 1 (PLGD). Ryplazim is a plasma-derived human plasminogen concentrate. This means it was made from donated human blood. For most patients, this medicine can be given through a needle in a vein (also called an IV, or intravenous infusion). Some patients may need a port. A port is a small device that goes under your skin, usually on your chest. Ports are used to draw blood or to give treatments, like Ryplazim. Your doctor will talk to you about the best option for you.
Learn more about Ryplazim here.
Who can take Ryplazim?
Ryplazim was tested in clinical trials in patients aged from 4 to 42 years. The FDA approved Ryplazim for patients of all ages.
What were the most common side effects in the clinical trials?
These side effects were seen in more than 10 percent of people in clinical trials:
Stomach pain, feeling sick to your stomach, or bloating
Fatigue (feeling tired)
Pain in arms or legs
Hemorrhage (bleeding)
Constipation (hard poop that you may have trouble passing)
Dry mouth
Headache
Feeling dizzy
Joint stiffness
Back pain
I am a patient with plasminogen deficiency type 1 (PLGD). What do I need to do to start this medicine?
- For US patients: Your healthcare provider can complete the the “New Start Form” and fax it to the number provided on the form. We encourage HCPs seeking additional information or guidance on how to complete the Enrollment Form/New Start Form to contact a Rare Disease Specialist by using this form.
- For patients outside of the US: Ryplazim is currently unavailable to patients outside the United States, as Ryplazim is currently only approved in the US. However, for patients with serious or life-threatening conditions, HCPs may reach out to Kedrion’s Global Medical and Scientific Service at MedInfo@kedrion.com .
Why should I get care from a Hemophilia Treatment Center (HTC)?
We encourage you to get care from your nearest Hemophilia Treatment Center (HTC), where there are doctors who understand PLGD. The doctors at an HTC can work with you and with Kedrion to get Ryplazim and teach you how to use it.
There are lots of reasons to get care from the closest HTC:
- HTCs provide patients with the support you need to understand and live with your diagnosis. HTCs have a multidisciplinary team. This team includes social workers who can help you with your school and work needs.
- HTCs have experience working with insurance companies to get approval for the care you need.
- The doctors at an HTC are trained to look at the whole body in PLGD patients. They don’t just look at the areas where you have symptoms.
- HTCs understand how to use clotting factor concentrates. They know when and how to adjust your dose.
- HTCs know how what to do if you need surgery or if you develop new symptoms from your plasminogen deficiency.
- HTCs can teach you how to give yourself your medicine, and how to keep a record of how much you use.
How do HTCs improve care for people with PLGD?
HTCs are part of a national network that collects important data on people with plasminogen deficiency. This data is used to improve your care and treatment. The data doesn’t include any personal information, so your identity is always kept safe. There are a lot of things we still don’t know about plasminogen deficiency. By working together through this national network, we can learn more.
Here are some questions that the national network is trying to answer:
Why do some people develop symptoms and others don’t?
Why do some people have symptoms for a long time and others only have symptoms for a short time?
Although Ryplazim has been studied and FDA approved, we can still learn even more about how it helps individual people. HTCs may also have other studies that you could be part of. These studies might improve how we understand and care for people with plasminogen deficiency.
Will someone teach me how to give myself this medicine? What if I can’t do it?
The nursing staff at your HTC will teach you to give yourself Ryplazim at home. The staff at your HTC will continue to teach and support you until you are able to give yourself the medication at home.
Will Ryplazim be covered by my insurance?
Kedrion Biopharma is working with insurance companies to make sure Ryplazim will be covered. We expect that most insurance companies will cover this medicine.
Will there be any support to help with the costs for the out-of-pocket expenses?
Kedrion Biopharma is working with a patient support group to help patients with their out-of-pocket expenses if needed.